History
HIV treatment has come a long way since 1987 when the first breakthrough zidovudine, a failed cancer drug from the 1960’s, was found to stop HIV from multiplying and help people with HIV live longer. The problem with a single drug treatment for HIV is that viruses mutate over time and these mutations eventually cause the drug to stop working. Antiretroviral drugs that overcome mutation and resistance by attacking the virus differently have been discovered and approved throughout the years. There are currently seven major classes of antiretroviral drugs which include the nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), fusion inhibitors, CCR5 antagonists, post-attachment inhibitors, and integrase strand transfer inhibitors (INSTIs). In 1996 it was discovered that combining certain HIV drugs could suppress the virus’ replication and allow an HIV patient’s immune system to recover and to fight off infections like pneumonia. This breakthrough in combination antiretroviral therapy (ART) has made HIV a manageable and now considered by many a chronic illness instead of an untreatable disease. ART is not a cure, but it can help people with HIV live longer and healthier lives.
The Latest
New Long-Acting Treatment Option
Early on, the daily pill burden was quite cumbersome. STRs (Single Tablet Regimens) allowed HIV patients to take a single, once-a-day, fixed-dose tablet combining multiple drugs. Making compliance easier for patients while reducing side effects. Recently the FDA approved Cabenuva, the first long-acting drug combo for HIV, which is administered IM (intramuscularly) monthly in two separate shots.
Cabenuva is approved to treat HIV-1 infection in adults with no history of treatment failure and who have not shown signs of resistance to the two drugs in Cabenuva. Developed to replace the patient’s current oral antiretroviral regimen, Cabenuva comes in a kit that consists of two separate injections of cabotegravir (INSTI) and rilpivirine (NNRTI), a complete regimen to treat HIV-1 infection. Before starting the monthly Cabenuva injections, the patient must take approximately one month (at least 28 days) of the oral lead-in drugs (cabotegravir and rilpivirine) to see how well they tolerate these medicines. Oral Edurant (rilpivirine) 25mg tablets have been commercially available for years and the FDA recently approved Vocabria (cabotegravir) 30mg tablets. An initiation injection of Cabenuva is administered on the last day of the oral lead-in dose. This initiation injection is dosed higher with Cabotegravir 600mg and Rilpivirine 900mg.
See table below for dosing and cost.
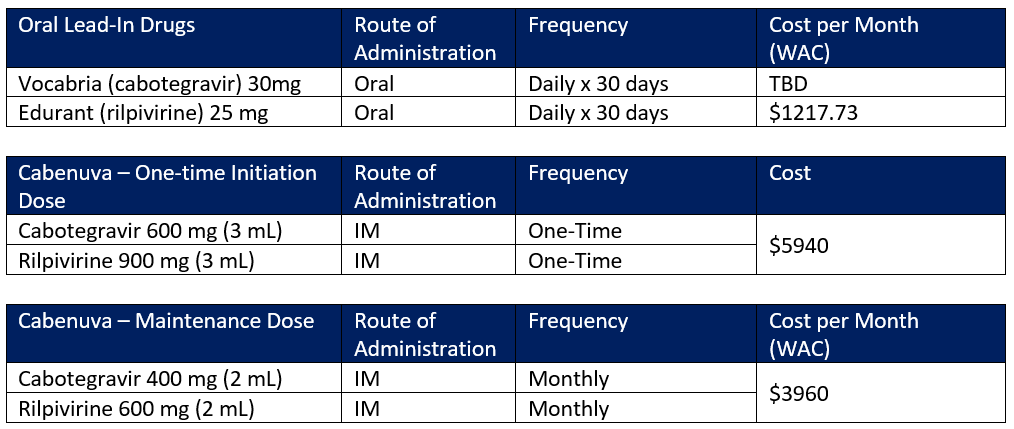
Cabenuva is an intramuscular injection and must be administered by a healthcare professional and will most likely be covered under the medical benefit. Cabenuva will compete with single-tablet regimens (STRs) for the treatment of HIV infections. The company has publicly stated that Cabenuva is “within the range” of what STRs cost now, but our research shows that the cost of Cabenuva is in the upper limits of the SRT “range”.
Due to the high cost of the medication and to ensure appropriate use, payers should consider prior authorization. If covered under the pharmacy benefit, quantity limits and coverage on specialty drug tiers should also be considered. The real world benefit of treating HIV patients with a monthly injectable medication over a once daily one pill regimen must be established. Adherence may improve with certain patient populations such as people with mental illness or substance abuse issues; however, scheduling monthly doctor’s visit along with the costs associated with these visits will factor into determining how this drug will improve adherence.
New Generic
Approximately two million new HIV cases occur yearly worldwide. There is no vaccine available to prevent HIV transmission, therefore, behavioral and biomedical HIV prevention strategies are needed to reduce the spread of HIV. For high-risk patients not infected with HIV, Pre-Exposure Prophylaxis (PrEP) using antiretroviral medications is an evidence-based approach. Teva launched a generic version of Gilead’s Truvada (emtricitabine and tenofovir disoproxil fumarate) on October 2, 2020. Truvada is a STR approved for PrEP as well as the treatment of HIV-1 infection. This is the first generic product available for PrEP. The only other drug with the PrEP indication is Descovy (Emtricitabine/Tenofovir Alafenamide Fumarate) which is also manufactured by Gilead. The cost of brand Truvada 200-300mg is $1,842.28 (WAC) for a 30-day supply compared to $1445.40 (WAC) for a 30-day supply of generic emtricitabine and tenofovir disoproxil fumarate 200-300mg. As prices continue to come down, some payers have already moved to restrict Descovy for PrEP. There may be exceptions for patients with high risk of bone or renal disease where Descovy has shown to have less safety issues in these patient populations.
The treatment of HIV has come a long way since 1987. HIV has turned into a chronic disease. Advances and SRT’s have made taking these medications, which needs to be taken for life, less daunting. Some of the older medications have generic alternatives which may help manage the cost of HIV. We still do not have a cure, but the pipeline is robust. We now have many treatment options to choose from that allow people that are HIV positive live a longer and healthier life.







